APPLICATIONS:
- Work with acid media
- Battery testing and maintenance
- Corrosion testing
- Electro-analytical studies
- Measure half-cell potentials in lead-acid batteries
- Battery testing and maintenance
- Corrosion testing
- Electro-analytical studies
- Measure half-cell potentials in lead-acid batteries
STEPS TO VERIFY VIABILITY OF ELECTRODE:
- Create a ~5mm layer of electrode solution in a beaker
- Place two electrodes of the same type into the pool of solution
- Compare the readings using a volt meter
- If they are within 10 mV of variation, it is good to go!
FEATURES:
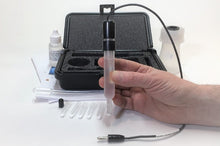
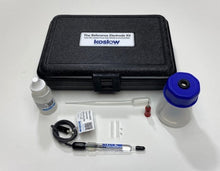
- Carry Case
- Electrode Refill Solution Electrolyte 30 ml
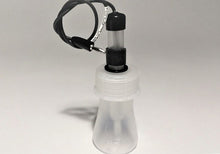
- Laboratory Electrode Storage Bottle
- Pack of 2 Replacement Porous Tips
- 2 Heat-Shrinkable Sleeves
- Electrode Filling Pipette
- Absorbent Foam
- Maintenance Manual
- Our standard off-the-shelf concentration is 3.8 Molar (Specific Gravity of 1.215)
- You can order your electrode with your specified acid molarities, minimize junction potentials, and perform calculations.
- Prepared with reagent-grade mercury sulfate and H2SO4 solution.
- Electrode potential is stable and recovers quickly from small temperature excursions.
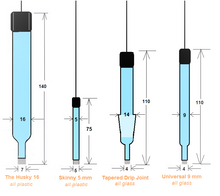
- We can fabricate your electrode's body to your custom specs.
- The porous junction tip is made with electroporous KT Glass and is replaceable when damaged or contaminated.
- Low noise, low impedance, Prime ion transfer
- Traceable ID tags on the lead wire
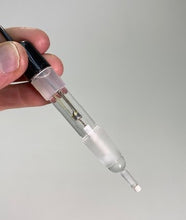
- The Hg/HgSO₄ reference electrode uses a filling solution of sulfuric acid (Part No. 3021). Ready-to-use reference electrode. All Koslow electrodes are fully assembled and filled.
LEARN MORE:
- Electrode Only (No Kit)
- Reference Electrode Accessories
- Promotional Flyer
- Mercury Sulfate Electrode standard potentials
- Select the Right Koslow Reference Electrode
- What are Reference Electrodes?