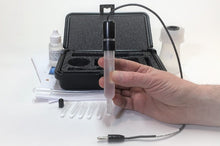
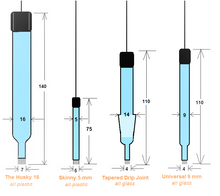
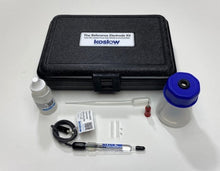
The silver/silver chloride reference electrode is many electrochemists' reference electrode of choice. It is widely used, stable, non-toxic, and quite robust. The silver-silver chloride electrode's simplicity and ruggedness make it the right candidate for many commercial applications where the electrochemical potential must be measured or controlled. It is mainly used with saturated potassium chloride (KCL) electrolytes but can also be filled with lower concentrations, such as 1 M KCL, and even directly in seawater. This kit includes the Reference Electrode with compatible maintenance and refill accessories.
Potentials:
- 244 mV vs. Normal Hydrogen Electrode (NHE)
- 410 mV vs. Mercury Sulfate Reference Electrode (M.S.E.)
- 440 mV vs. Silver / Silver Sulfate
STEPS TO VERIFY VIABILITY OF ELECTRODE:
- Create a ~5mm layer of electrode solution in a beaker
- Place two electrodes of the same type into the pool of solution
- Compare the readings using a volt meter
- If they are within 10 mV of variation, it is good to go!
APPLICATIONS:
- Battery testing and maintenance
- Corrosion testing
- Electro-analytical studies
FEATURES:
- Carry Case
- Electrode Refill Solution Electrolyte 30 ml
- Laboratory Electrode Storage Bottle
- Pack of 2 Replacement Porous Tips
- 2 Heat-Shrinkable Sleeves
- Electrode Filling Pipette
- Absorbent Foam
- Maintenance Manual
- The porous junction tip is made with electroporous K.T. Glass and is replaceable when damaged or contaminated
- Excellent ion transfer and low noise
- Traceable ID tags
- Instructions with S.D.S.
HOW IT WORKS:
The Silver-Silver Chloride electrode has been shown to possess superior characteristics to that of non-chloride silver electrodes when recording low-level A.C. and D.C. potentials. Chloridized silver electrodes present less low-frequency "noise" than gold or silver electrodes. The Koslow Silver-Silver Chloride electrodes are constructed of pure silver and then chloridized to provide stability and low noise characteristics. The Silver/Silver Chloride potential is approximately -0.199 Volts vs. Normal Hydrogen Electrode (NHE) or -0.047 Volts vs. Saturated Calomel Reference Electrode (S.C.E.) Electrode potential is stable to plus or minus one millivolt and recovers quickly from small temperature excursions.
Koslow reference electrodes use a saturated KCL solution with excess KCL crystals. These can sometimes be seen on the bottom of the electrode. The extra KCL dissolves into the electrolyte as the potassium and chloride ions diffuse through the liquid junction regularly. This additional buffer of KCL extends the time before the reference cell starts to drift due to the depletion of chloride ions in the electrolyte.
Aging: U.V. light decomposes AgCL to give silver(0), giving the electrode a black appearance. Standard fluorescent lamps should be delicate, but don't store your references in direct sunlight. Light-protected amber glass storage bottles are recommended for storing electrodes with frit in KCL filling solution. Saturated solutions of KCL have the advantage that the concentration is reproducible even if the temperature changes (if solid salt is present) and are immune to the effects of water.
The Electro porous K.T. Glass tip is the ionically conducting electrical pathway between the inside of the reference electrode and the bulk of your cell. The Electro-porous KT Glass frit (about 1/8" long) is attached to the glass tube by a heat-shrinkable Teflon sleeve. The Glass has a low electrical resistance (under ten kilohms for the standard filling solutions) and a modest leak rate. The electrical resistance of the reference electrode 'frit' is an essential factor in determining the stability and speed of your potentiostat in actual use. When changing K.T. Glass frits, the heat-shrink tubing should be trimmed flush with the end of the K.T. Glass frit to prevent the capturing of air bubbles.
LEARN MORE:
- Electrode Only (No Kit)
- Reference Electrode Accessories
- Promotional Flyer
- Select the Right Koslow Reference Electrode
- What are Reference Electrodes?