The Silver/ Silver Sulfate Probe or the Silver Sulfate Reference Electrode is a chloride-free, mercury-free alternative to the Mercury-based electrodes.
A new reference electrode for the 21st century
Features:
-
Use for chloride-free investigations
-
Stable reference
-
Internal Filling: silver sulfate and saturated potassium sulfate
-
Easily disposable, contains no mercury
-
Replaceable porous frit
Ag/Ag2S04 Reference Electrode potential:
+0.686 Volts vs. Normal Hydrogen Electrode (NHE)
+0.038 Volts vs. Mercury Sulfate Reference Electrode (M.S.E.)
+0.440 Volts vs. Saturated Calomel Reference Electrode (S.C.E.)
It is genuinely environmentally green since it does not contain mercury. The Silver Sulfate Electrode is non-hazardous. It is fabricated with pure silver and potassium sulfate electrolyte. The silver-silver sulfate electrode's simplicity and ruggedness make it a good candidate for many commercial applications where the electrochemical potential must be measured or controlled.
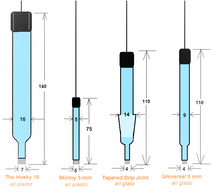
Please choose from our four body shape designs or provide your custom design. We can fabricate your electrode's body to your specs.
Electrode potential is stable to plus or minus one millivolt and recovers quickly from small temperature excursions. The Silver Sulfate Electrode will find applications in electro-analysis, battery testing and maintenance, corrosion testing, and electro-analytical studies. The electrodes are assembled using pure silver and then anodized to provide stability and low noise characteristics.
The junction is either Electro-Porous KT Glass or a plastic Wick Stick that serves as the ionically conducting electrical pathway between the inside of the reference electrode and the bulk of your cell. The Electro-porous KT Glass frit is attached to the glass tube by a heat-shrinkable Teflon sleeve. The K.T. Glass has a low electrical resistance and a modest drip-rate. The electrical resistance of the reference electrode frit is an essential factor in determining the stability and speed of your potentiostat's actual use.
The Silver Sulfate Electrode uses a saturated potassium sulfate solution with excess silver and potassium sulfate crystals. These can sometimes be seen on the bottom of the electrode. The extra crystals dissolve into the electrolyte as the ions diffuse through the liquid junction in everyday use. This additional buffer of K₂SO₄ extends the time before the reference cell starts to drift due to the depletion of potassium ions in the electrolyte. Lead-acid battery investigations and other possible uses of the Silver/ Silver Sulfate Reference ELectrode include the determination of individual capacities of positive and negative plates, monitoring individual electrode behavior during deep discharge and cell reversal, optimization charge or discharge parameters by controlling the current such that pre-determined limits of positive or negative half-cell potential are respected, observation of acid concentration differences, for example, due to acid stratification, by measuring diffusion potentials (concentration-cell voltages) and detection of defective cells and defective plate sets, in a string of cells, at the end of their service life.
Learn More: https://youtu.be/oKnZIq5a38I