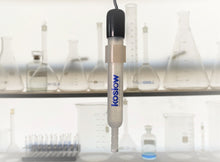
Non-Aqueous Reference Electrode Kit - 1006.
The Universal 9 glass body with a banana jack is our standard and is always in stock.
Contains:
One non-chloridized silver wire electrode: Removable top cap, electrode tube assembly, electro-porous K.T. glass tip, belch hole with closure.
A vial (1006VG) contains 1 gram of Silver Nitrate powder
(Serial number #1006-VG)
It is packaged inside a press seal bag, pink foam, and a mailer carton with S.D.S.
PLEASE NOTE: NO LIQUID. The customer is to supply solvent.
I need an anhydrous electrolyte, so an aqueous reference electrode is unsuitable. What are the alternatives?
If contamination by water from aqueous electrodes is a problem, several alternatives exist. The simplest is to use a salt bridge (Koslow Part No. S.B.) containing the anhydrous electrolyte to separate the aqueous reference electrode from the analyte solution. Other alternatives include using a non-aqueous reference. The Koslow non-aqueous reference electrode (Part No 1006) requires user assembly. It consists of a silver wire immersed in a solution containing silver nitrate dissolved in an appropriate electrolyte solution. Ideally, this electrolyte is the same as used for the analyte (to eliminate junction potentials); an acetonitrile-based electrolyte can generally be used. The potential of the non-aqueous reference electrode depends on the solvent, the electrolyte, and the concentrations of silver nitrate and salt. Since the potential of a non-aqueous reference electrode can vary among different electrodes, redox potentials measured using such a reference electrode should be quoted relative to an internal reference compound (e.g., ferrocene). A pseudo-reference electrode is a platinum or silver wire immersed in the analyte solution. This has the advantage that there can be no contamination of the analyte. Still, the disadvantage is that the reference potential is unknown, as it depends on the analyte solution's composition. Therefore, redox potentials measured using a pseudo-reference electrode should again be quoted relative to an internal reference compound such as ferrocene.
Learn More: